Thalidomide was marketed in 1956 by Chemie Grünenthal in Western Germany, first as an anti-flu, then in 1957, as an hypnotic drug. It was then available without prescription. In April 1958, thalidomide was marketed in the United Kingdom by Distillers Company. Several countries followed suit and thalidomide was put into circulation under many different brands. Overall, thalidomide was sold under about 40 different names around the world, principally in Western countries and in Japan. Important advertising campaigns were led by its fabricants, starting with Chemie Grünenthal and Distillers Company. Thalidomide was described as a miracle drug. Thousands of samples were distributed to doctors, who were encouraged to prescribe it to pregnant women in order to alleviate pregnancy nausea. Everyone was told that this drug represented no risk at all for pregnant women.
The following excerpt, from the website of the documentary “NO Limits” addressing the thalidomide tragedy, describes particularly well how negligent Grünenthal was regarding the safety of thalidomide:
“What the public did not know is that Grünenthal had no reliable evidence to back up its claims that the drug was safe. They also ignored the increasing number of reports coming in about harmful side-effects as the drug was being used. In fact, starting in 1959 Grünenthal was flooded with complaints from doctors about mild to severe and sometimes permanent nerve damage, especially by elderly people who had used the drug as a sleeping aid.
[…]
The company was equally dismissive of concerns related to deformed babies. The drug was widely promoted as an anti-nausea drug for pregnant women experiencing morning sickness. When the company was confronted with reports on malformed babies and suggestions that the malformations could be possibly linked to Thalidomide, they didn’t react. Instead of taking all those reports seriously Grünenthal responded with measures to keep the drug on the market.”
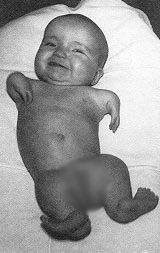
As early as 1960, unsuspected side effects on the nervous system started to be attributed to thalidomide by some doctors. The first concerns about teratogenic hazards were raised in Western Germany in October 1961. We had to wait more than six weeks after that for the drug to be withdrawn from the british and german markets, at the end of November and in early December. But it was already too late: thousands of babies around the world would be born with severe malformations. Other authorities were even slower to withdraw thalidomide from the market, so that in some countries, it was available until the end of 1963.
It is hard to tell with precision how many thalidomide victims there is, because a lot of babies were dead before birth, stillborn or died soon after birth due to the severity of their malformations. Not all of these births were registered in proper form, especially considering that several thalidomiders infants are believed to have been infanticide victims. It is estimated that 15,000 children were born worldwide with malformations attributable to thalidomide. The victims also include the families of all these children, whose life were severely impacted by this tragedy.
Not so long after that, new therapeutical effects were discovered to thalidomide, for treating or alleviating leprosy, systemic lupus erythematosus and some cancers, among others. The drug is currently available in many countries for these uses. To know more about the actual uses of thalidomide, click here.